Novel Insights. Novel Therapies.
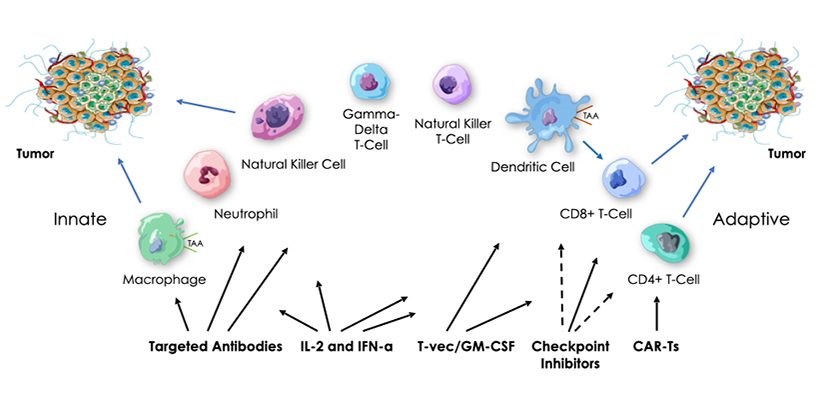
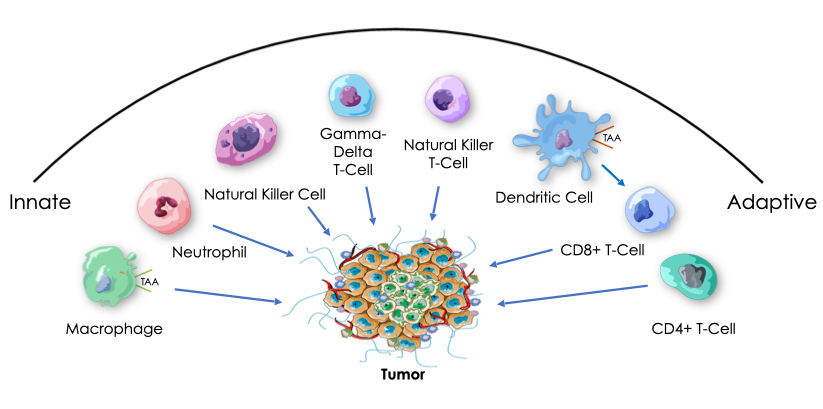
Historically, we know that tumor regression has been observed in the presence of bacterial infection. We also know that bacteria contain immune system danger signals, called pathogen-associated molecular patterns (PAMPs), that collectively can activate all of the cellular components of our innate and adaptive immune pathways. PAMPs are recognized by receptors, such as Toll-like (TLR), NOD, STING and RIG-I, that are found on and involved in activation of many different innate and adaptive immune cells.
Our platform is based on the hypothesis that highly efficient anti-tumor immunotherapy will require safe activation of both innate and adaptive cellular immunity in both tumors and immune organs, and that this might be achieved with a multi-targeted package of bacterial PAMPs, in the form of attenuated and killed, intact but non-pathogenic bacteria delivered intravenously. While current therapies are increasingly becoming more and more personalized and costly, we are advancing an approach designed to be widely accessible, with broad anti-tumor and anti-viral activity not dependent on the targeting of specific tumor or viral antigens.
Our Unique Approach
Previous research has shown that lipopolysaccharide (LPS), an endotoxin that binds to Toll-like receptor 4 (TLR4), is a key bacterial PAMP that activates the immune system. Activated TLR4 has been shown to play a role in dendritic cell activation and T-cell-mediated anti-tumor immune responses. Our novel insights have enabled us to create attenuated and killed, non-pathogenic gram-negative bacteria with unique levels of LPS – levels that have now been shown in pre-clinical studies to be sufficient to synergize with other PAMPs in the bacteria to safely prime and/or activate innate and adaptive immune pathways. We currently have a broad patent portfolio with 34 issued or granted patents that are based on the technology originally developed by our Founder and Chief Scientific Officer, Dr. Michael Newman, at Indaptus’ predecessor company, Decoy Biosystems.
Based on our successes to date, we are now building a pipeline of therapeutic candidates designed to be delivered intravenously, targeting cancers and infectious diseases with high unmet medical needs.
Results to Date
We are currently advancing our lead candidate, Decoy20, through Phase 1 clinical trial. To date, Decoy20 and/or related candidates have demonstrated broad anti-tumor and anti-viral activity in pre-clinical models, including high percentage complete and durable anti-tumor responses in combination with different classes of existing therapeutics.
- In oncology, Decoy candidates have demonstrated the ability to eradicate established tumors in a murine model of hepatocellular carcinoma in combination with either a non-steroidal anti-inflammatory drug (NSAID) or an anti-PD-1 agent, and more efficiently with both. Tumor eradication has occurred with a wide therapeutic index and has led to induction of 100% immunological memory. In combination with low-dose chemotherapy, Decoy candidates have also produced highly efficient eradication of established tumors in a mouse model of non-Hodgkin’s Lymphoma (NHL), also with induction of immunological memory. Combination-mediated tumor eradication has also been observed with a human tumor xenograft NHL model with inclusion of a targeted antibody. Decoy candidates have also produced significant single agent activity in murine models of both metastatic pancreatic carcinoma and orthotopic, colorectal carcinoma.
- In infectious disease, single agent Decoy therapeutics have produced significantly broader activity than standard of care treatment in a pre-clinical model of chronic Hepatitis B infection, as well as single agent activity against chronic HIV infection in a pre-clinical humanized mouse model.
Generation and/or activation of the cells required for innate and adaptive anti-tumor and anti-viral immune responses takes place, to a significant extent, outside of the tumor or sites of infection, including in the spleen. Our intravenous therapeutic candidates are expected to passively target the liver, spleen, and leaky vasculature of tumors, producing immune activation in an immune organ, as well as a common site for primary and metastatic cancer and HBV infection, the liver. As our therapeutic candidates are expected to be cleared very quickly by the liver and spleen, we anticipate a low risk of non-specific autoimmune side effects relative to other types of immunotherapies designed for continuous exposure.
We have initiated our Phase 1 clinical trial of Decoy20 in December 2022 and dosed our first patient in March 2023.
Key Milestones
First dosing of Decoy20 in Q1 2023
Initial single dose safety data 2H 2023
Multi-cohort single dose safety data 1H 2024
Multi-dose safety data 2H 2024
Combo Data Proof of Concepts in late 2025 / early 2026
Publications and Presentations
September 12, 2025 – CICON25
November 7, 2024 – Frontiers in Immunology
November 6-10, 2024 – Society for Immunotherapy of Cancer (SITC)
May 31-June 4, 2024 – American Society of Clinical Oncology (ASCO)
April 5-10, 2024 – AACR Poster Presentation
November 1-5, 2023 – Society for Immunotherapy of Cancer (SITC)
May 9-11, 2023 – 4th STING & TLR Targeting Therapies Summit
- Pre-Clinical Anti-HBV Activity with a Passively Targeted, Multi-TLR, NOD and STING Agonist
- Breaking the Toxicity Barrier: Tumor Eradication in Pre-Clinical Models by Systemic Administration of a Multi-TLR, NOD and STING Agonist
April 2023 – AACR Poster Presentation
- A systemically administered killed bacteria-based immune receptor agonist for pulsed anti-tumor immunotherapy
April 25-27, 2022 – Chronic HBV Drug Development
- Driving Pre-Clinical Anti-HBV Activity With a Novel Multi-TLR Agonist Therapeutic Vaccine
STING & TLR-Targeted Therapies Summit 2022
- Eradication of Established Tumors with Induction of Innate & Adaptive Immunological Memory in Multiple Preclinical Models with Systemically Administered Decoy Bacteria, a Multi-TLR Agonist Therapeutic Vaccine
May 25-27, 2021 – STING & TLR-Targeting Therapies Summit – Virtual Presentation
- Combination Systemic Therapy with a Multiple TLR Agonist Safely Eradicates Established Tumors with Induction of Innate and Adaptive Immunological Memory
2018 Meeting of CRI-CIMT-EATI-AACR – Poster Presentation
- Development and pre-clinical efficacy characterization of a systemically administered multiple Toll-like receptor (TLR) agonist for anti-tumor immunotherapy