Indaptus Therapeutics Reports Second Quarter 2022 Financial Results and Provides Corporate Update
August 8, 2022
U.S. Food and Drug Administration (FDA) Cleared Investigational New Drug (IND) Application for Decoy20
On Track to Initiate Phase 1 Clinical Trial of Decoy20 for Treatment of Solid Tumors in 2022
NEW YORK (August 8, 2022) – Indaptus Therapeutics, Inc. (Nasdaq: INDP) (“Indaptus” or the “Company”), today announces financial results for the second quarter ended June 30, 2022 and provides a corporate update.
“We made substantial progress throughout the second quarter, highlighted by the FDA clearance of our IND application for systemically administered Decoy20. This important regulatory milestone keeps us on track to initiate our first in human clinical trial this year,” said Jeffrey Meckler, Chief Executive Officer of Indaptus. “Despite advances in the immuno-oncology field, there still remains a great unmet need in the treatment of solid tumor cancers. We believe Decoy20 has the potential to effectively tackle these hard-to-treat tumors with its ability to activate both the innate and adaptive immune systems.”
Recent Corporate Highlights
Investigational New Drug Application Clearance
In May, Indaptus announced that the FDA cleared the Company’s IND application for a Phase 1, open-label dose escalation and expansion, clinical trial in patients with advanced solid tumors where currently approved therapies have failed. The study is designed to evaluate the safety, tolerability, and preliminary efficacy of Decoy 20 and will follow a 3+3 design of dose-escalation cohorts. The study protocol allows for exploration of additional dosing regimens, including continuous weekly administration after initial safety has been established. Decoy20 has the potential to treat a wide range of solid tumors including hepatocellular, colorectal and pancreatic carcinomas.
Earlier pre-clinical data have demonstrated Decoy20’s ability to eradicate established tumors in a murine model of hepatocellular carcinoma in combination with either a non-steroidal anti-inflammatory drug (NSAID) or an anti-PD-1 agent, and more efficiently with both. Tumor eradication has occurred with a wide therapeutic index and has led to induction of 100% immunological memory. Mechanism of actions studies have demonstrated activation of innate and adaptive immune pathways and immunologically cold to hot transition in subcutaneous tumors after only one intravenous (IV) administration of Decoy product in the tumor eradicating, combination setting.
In combination with low-dose chemotherapy, Decoy candidates have also produced highly efficient eradication of established tumors in a mouse model of non-Hodgkin’s lymphoma (NHL), also with induction of immunological memory. Combination-mediated tumor eradication has also been observed with a human tumor xenograft NHL model with inclusion of a targeted antibody. Mechanism of action studies have demonstrated involvement of activation of both innate and adaptive immune pathways in this anti-tumor activity. Decoy20 has also produced significant single agent activity in murine models of both metastatic pancreatic carcinoma and orthotopic, colorectal carcinoma.
Financial Highlights for Second Quarter Ended June 30, 2022
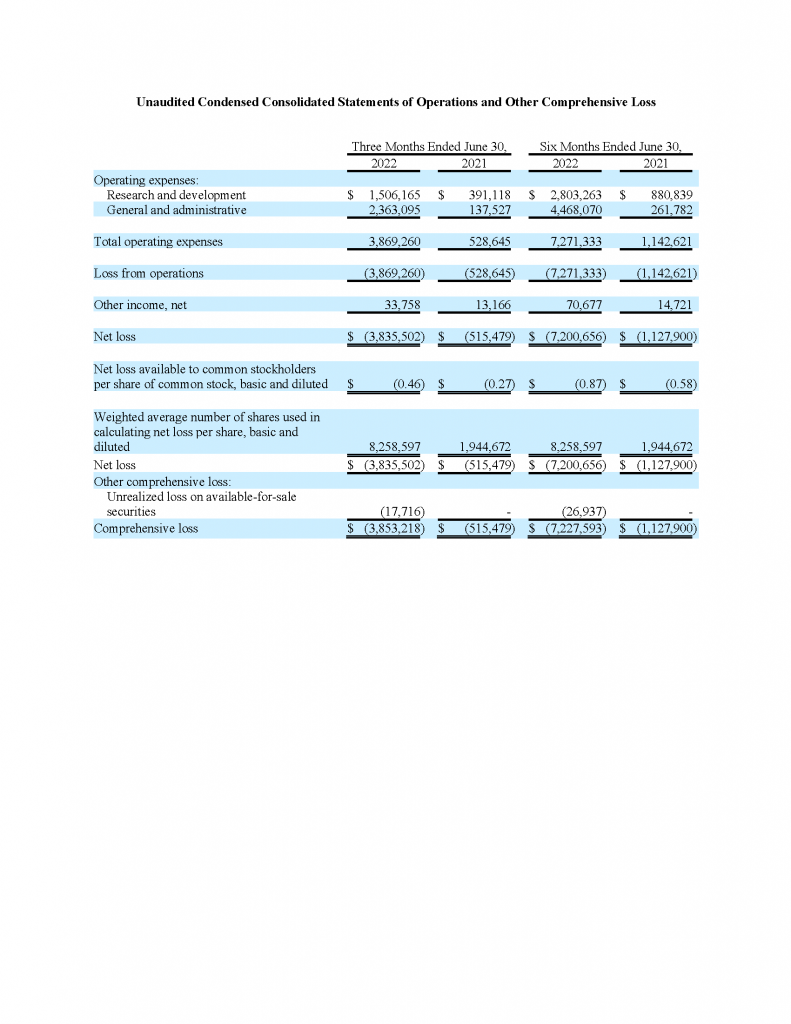
Research and development expenses, for the three-month period ended June 30, 2022, were approximately $1.5 million, an increase of approximately $1.1 million compared with approximately $390,000 in the three-month period ended June 30, 2021. Research and development expenses for the six-month period ended June 30, 2022, were approximately $2.8 million, an increase of approximately $1.9 million compared with approximately $900,000 in the six-month period ended June 30, 2021. The increase for the three and six-month periods was primarily due to payroll and related expenses including stock-based compensation, and the preparation of the Phase 1 clinical trial and IND submission.
General and administrative expenses, for the three-month period ended June 30, 2022, were approximately $2.4 million, an increase of approximately $2.2 million compared with approximately $138,000 in the three-month period ended June 30, 2021. General and administrative expenses for the six-month period ended June 30, 2022, were approximately $4.5 million, an increase of approximately $4.2 million compared with approximately $260,000 in the six-month period ended June 30, 2021. The increase for the three and six-month periods was primarily due to payroll and related expenses, including stock-based compensation, resulting from increased headcount of our executive team following the Merger and increase in directors’ and officers’ insurance policy, professional fees and other expenses associated with being a public company following the Merger.
Loss per share for the three-month period ended June 30, 2022, was approximately $0.46 compared with approximately $0.27 for the three-month period ended June 30, 2021. Loss per share for the six-month period ended June 30, 2022, was approximately $0.87 compared with approximately $0.58 for the six-month period ended June 30, 2021.
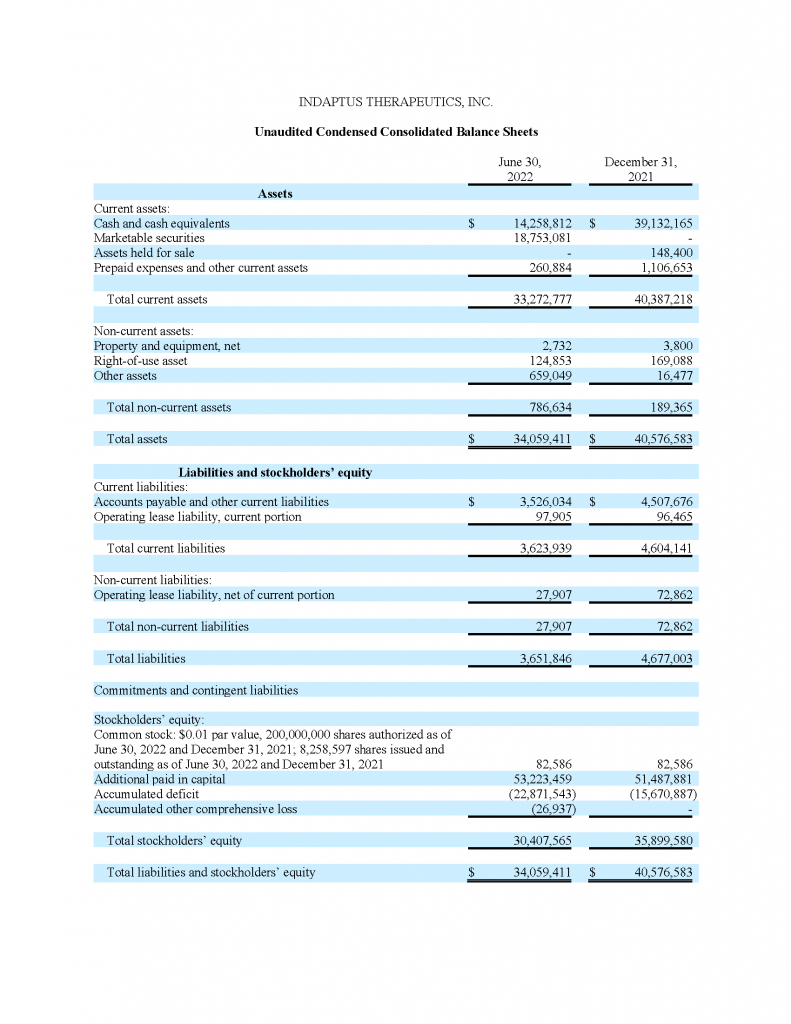
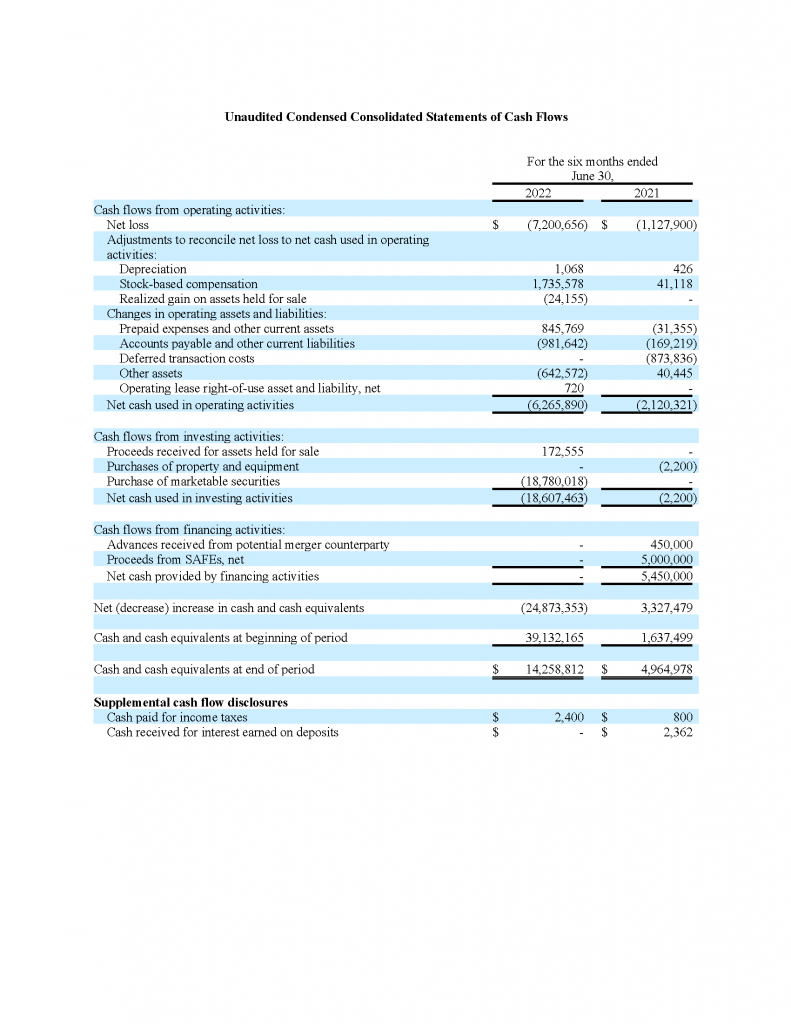
As of June 30, 2022, the Company had cash, cash equivalents and short-term investments of approximately $33.0 million. As of December 31, 2021, the Company had cash and cash equivalents of approximately $39.1 million.
Net cash used in operating activities was approximately $6.3 million for the six-month period ended June 30, 2022, compared with net cash used in operating activities of approximately $2.1 million for the six-month period ended June 30, 2021. This increase resulted primarily from an increase in our research and development expenses and general and administrative expenses.
Net cash used in investing activities was approximately $18.6 million for the six-month period ended June 30, 2022 which was primarily due to the purchase of marketable securities investments during that period. There was an immaterial amount in net cash used in investing activities in the six months ended June 30, 2021.
There was no net cash provided by financing activities in the six months ended June 30, 2022.
About Indaptus Therapeutics
Indaptus Therapeutics has evolved from more than a century of immunotherapy advances. The Company’s approach is based on the hypothesis that efficient activation of both innate and adaptive immune cells and associated anti-tumor and anti-viral immune responses will require a multi-targeted package of immune system activating signals that can be administered safely intravenously. Indaptus’ patented technology is composed of single strains of attenuated and killed, non-pathogenic, Gram-negative bacteria, with reduced i.v. toxicity, but largely uncompromised ability to prime or activate many of the cellular components of innate and adaptive immunity. Decoy20 represents an antigen-agnostic technology that has produced significant single agent activity against metastatic pancreatic and orthotopic colorectal carcinomas, single agent eradication of established, antigen-expressing breast carcinoma, as well as combination-mediated eradication of established hepatocellular carcinomas and non-Hodgkin’s lymphomas in standard pre-clinical models, including syngeneic mouse tumors and human tumor xenografts. Tumor eradication has been observed with Decoy products in combination with anti-PD-1 checkpoint therapy, low-dose chemotherapy or an approved targeted antibody. Combination-based tumor eradication produces innate and adaptive immunological memory, involves activation of both innate and adaptive immune cells and is associated with induction of innate and adaptive immune pathways in tumors after only one i.v. dose of Decoy product, with associated “cold” to “hot” tumor inflammation signature transition. IND-enabling toxicology studies have demonstrated safe i.v. administration, with no sustained induction of hallmarks of cytokine release syndromes, possibly due to passive targeting to liver, spleen and tumor, followed by rapid elimination of the product. Indaptus products have also produced significant single agent activity against chronic hepatitis B virus (HBV) and chronic human immunodeficiency virus (HIV) infections in pre-clinical models.
Forward-Looking Statements
This press release contains forward-looking statements with the meaning of the Private Securities Litigation Reform Act. These include statements regarding management’s expectations, beliefs and intentions regarding, among other things, our product development efforts, business, financial condition, results of operations, strategies, plans and prospects. Forward-looking statements can be identified by the use of forward-looking words such as “believe”, “expect”, “intend”, “plan”, “may”, “should”, “could”, “might”, “seek”, “target”, “will”, “project”, “forecast”, “continue” or “anticipate” or their negatives or variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical matters. Forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause our actual results to differ materially from any future results expressed or implied by the forward-looking statements. Many factors could cause actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including, but not limited to, the following: our plans to develop and potentially commercialize its technology, the timing and cost of our planned investigational new drug application and any clinical trials, the completion and receiving favorable results in any clinical trials, Indaptus’ ability to obtain and maintain regulatory approval of any product candidate, our ability to protect and maintain its intellectual property and licensing arrangements, our ability to develop, manufacture and commercialize its product candidates, the risk of product liability claims, the availability of reimbursement, the influence of extensive and costly government regulation, and our estimates regarding future revenue, expenses capital requirements and the need for additional financing. More detailed information about the risks and uncertainties affecting us is contained under the heading “Risk Factors” included in our most recent Annual Report on Form 10-K filed with the SEC on March 21, 2022, and in other filings that we have made and may make with the Securities and Exchange Commission in the future. All forward-looking statements speak only as of the date of this press release and are expressly qualified in their entirety by the cautionary statements included in this press release. We undertake no obligation to update or revise forward-looking statements to reflect events or circumstances that arise after the date made or to reflect the occurrence of unanticipated events, except as required by applicable law.
Investor Contact:
Will O’Connor
Stern IR
+1 212-362-1200
will@sternir.com